Shortage Mitigation
On Demand Pharmaceuticals helps mitigate shortages for customers through the use of AI-enabled tools that analyze market trends, identify at-risk medicines, and assist in response through the management of ODP’s supply chains.
Ability to create new formulations and medicines quickly and to pivot rapidly from one product on the formulary to another.
Technology that provides pharmacists transparency into the supply chain and enables real-time monitoring of quality and production.
Responsiveness to supply chain and pricing volatility by offering predictable production and pricing, and more efficient use of staff time and resources.
Quality management and production systems that use cutting-edge technologies to meet the highest quality standards and comply with state and federal regulations.

On Demand Pharmaceuticals helps mitigate shortages for customers through the use of AI-enabled tools that analyze market trends, identify at-risk medicines, and assist in response through the management of ODP’s supply chains.
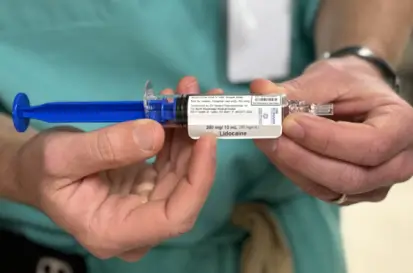
On Demand Pharmaceuticals, a drug manufacturer in the USA, offers rapid, high-quality production of 503A and 503B compounded medicines, addressing drug shortages with customizable, on-site solutions that ensure compliance, reduce waste, and enhance operational efficiency for healthcare providers and staff.

On Demand Pharmaceuticals is developing technology to ensure rapid access to critical medications during national emergencies with on demand, high-quality production solutions, addressing drug shortages to support healthcare facilities and save lives.

On Demand Pharmaceuticals’ innovative platform, an on demand pharmacy in the USA, is being developed to support the production of personalized medicines, clinical trial materials, small-scale CMO/CDMO operations, and generic drug manufacturing for pharmaceutical partners.
On Demand Pharmaceuticals Statement on the Rural Health Transformation Program
Rockville, MD – December 29, 2025 – On Demand Pharmaceuticals (ODP), a leader in advanced, on-demand pharmaceutical manufacturing, applauds the Trump Administration and the Centers for Medicare & Medicaid Services (CMS) for today’s historic announcement of $50 billion in funding awards to all 50 states through the Rural Health Transformation Program. Read moreOn Demand Pharmaceuticals Welcomes HHS Leadership to Showcase Breakthrough Domestic Pharmaceutical Manufacturing Capabilities
On Demand Pharmaceuticals (ODP) hosted senior officials from the U.S. Department of Health and Human Services (HHS) at its Centralized Production and Repackaging (CPR) facility in Rockville, Maryland. Read moreGinkgo Bioworks Teams Up with Partners on ARPA-H Project to Stabilize Pharmaceutical Supply Chains - Using Amber Waves of Grain
Ginkgo Bioworks and partners awarded $29 million contract by ARPA-H to develop distributed manufacturing of essential medicines using wheat germ cell-free expression systems Read moreSystem Director of Pharmacy

